Precision qEEG Biomarker Demonstrates Anxiolytic Efficacy in Phase 1 GRX-917 and Phase 1 Etifoxine
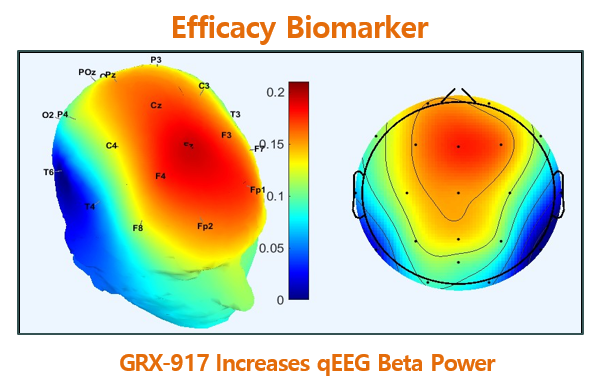
qEEG biomarkers demonstrated anxiolytic efficacy in both Phase 1 of GRX-917 and Phase 1 of etifoxine. qEEG Beta power increase Rapid-onset, sustained activity Time-dependent Exposure-dependent Increased qEEG Beta Power PD marker confirms GABAA receptor target engagement Anxiolytic efficacy biomarker For questions contact: Richard G. Farrell Chief Financial Officer Follow [...]