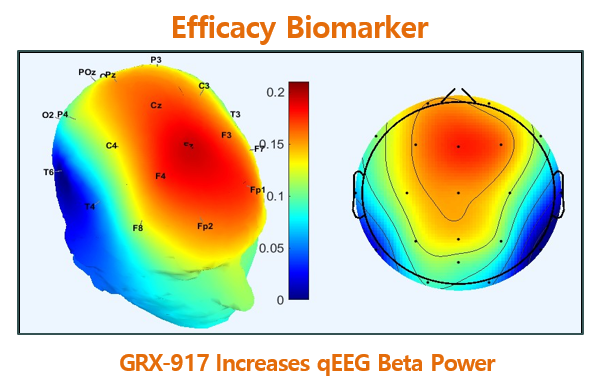
GRX-917 is a Potent Inhibitor of the NLRP3/IL-1 Beta Inflammation Pathway
Etifoxine (and therefore GRX-917) was recently shown to reduce neuroinflammation via inhibition of the NLRP3 inflammasome pathway (https://www.biorxiv.org/content/10.1101/2023.09.19.558428v1). Etifoxine inhibits the NLRP3 inflammasome pathway broadly, as shown by marked reductions of NLRP3, IL-1beta, and TNF. In addition, significant improvement in clinical scores were observed in the mouse EAE model of [...]