GENERALIZED ANXIETY DISORDER

Approvals in GAD Expected by 2029
GABA Therapeutics’ primary mission is to execute pivotal registration trials for GRX-917 in generalized anxiety disorder. GRX-917 is expected to be clinically superior to America’s top two anxiety drug classes: SSRIs/SNRIs and benzodiazepines, and without sedation, cognitive impairment, withdrawal or abuse. Trials will commence in 2026 with approvals in GAD expected by 2029.
Anxiety – Large Unmet Medical Need
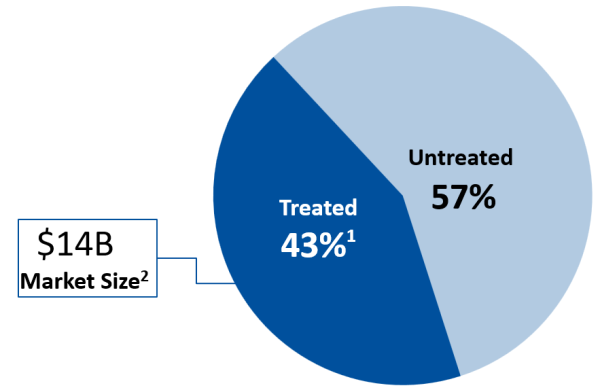
Anxiety disorders are the most common mental illness in the United States, affecting 40 million adults, or 19% of the U.S. population. Yet only 43% of generalized anxiety disorder patients receive treatment. This represents one of America’s largest unmet medical needs, primarily due to the limitations of U.S. anxiety medications.
1) Anxiety and Depression Association of America (2024)
2) Global Anxiety Market: IMS, per Foster Rosenblatt Market Research
Limitations of U.S. Anxiety Medications
The top 10 U.S. anxiety drugs fall into one of two drug classes:
1) SSRIs/SNRIs – the first-line anxiety drugs, take 4-6 weeks to take effect and are not effective in over 50% of patients.
2) Benzodiazepines – which are fast and effective, but have severe side effects and are involved in 31% of U.S. fatal prescription overdoses.
This explains why over 24 million anxiety disorder patients do not receive proper treatment in the United States.
Source: Anxiety and Depression Association of America / (NIMH, 2017)
“Benzodiazepines are involved in 31% of U.S. fatal prescription overdoses.”
*Source: Centers for Disease Control and Prevention
GRX-917 For Anxiety
GRX-917 is a deuterated (metabolically improved) analog of etifoxine, an approved anxiety medication that has been sold in France and over 25 smaller markets for decades. Clinical studies have shown that etifoxine has comparable speed of onset and efficacy as leading benzodiazepines, but with minimal to no side effects. GRX-917 has an identical mechanism of action as etifoxine, but with improved pharmakodynamics. It follows that GRX-917 is safer and clinically superior to all anxiety medications, both on market and in development.
GABA Therapeutics is planning to develop GRX-917 for anxiety in the United States, Europe and other patented countries. GRX-917 will initially be developed for treatment of generalized anxiety disorder and then expanded into other indications. Based on the extensive, 35+ years of clinical experience with etifoxine and its excellent efficacy and safety profile, the development of GRX-917 in GAD is considered very low risk. Social anxiety disorder and panic disorder are also deemed low risk indications, given etifoxine’s known reduction in HAM-A score.

