GRX-917 PIPELINE
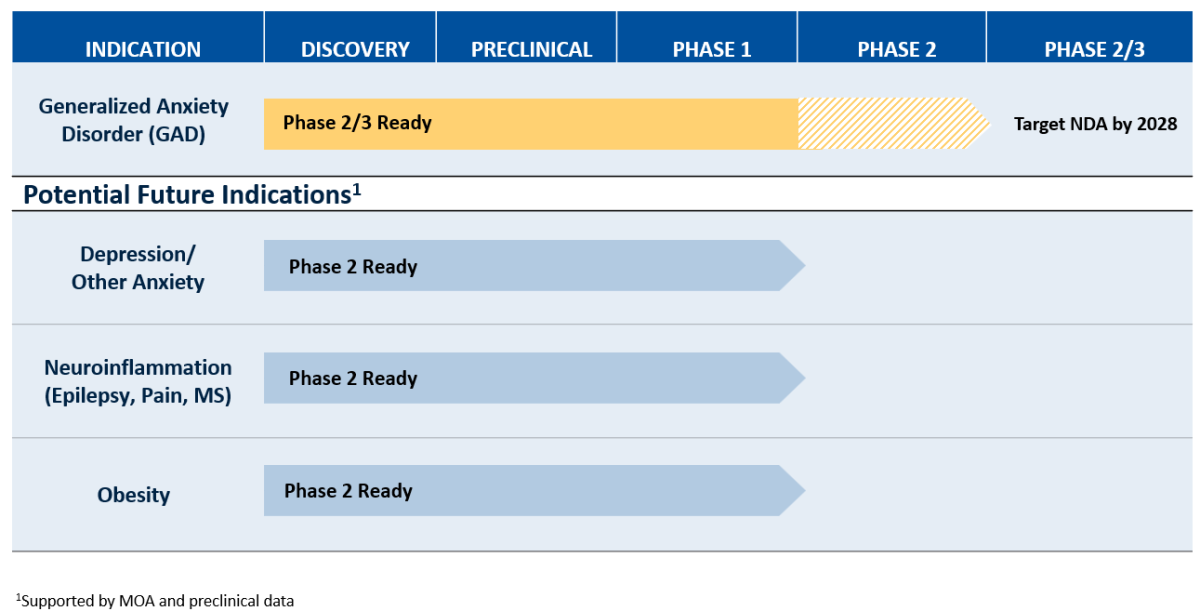
The primary clinical development focus is getting GRX-917 approved in generalized anxiety disorder by 2028/29, since GAD is a derisked indication with large unmet medical need. Phase 3 registration trials in GAD are planned to commence in 2026.
Additional large indications are supported by its mechanism of action and compelling preclinical efficacy data.